Tissue Biomechanics in the Whole Organism
Cell polarity and cell fate in the Drosophila female and male reproductive system: a Cell Biological and Biophysical approach
Our research group focuses on understanding the genetic, cell biology and biophysics basis of cell polarity and cell fate in a multicellular organism. Experimental biophysicists and biologists are welcome to join us.
We use Drosophila melanogaster as a model system to understand how cells adopt their fate in both the female and male germline, and how the oocyte is polarised. These are basic cellular processes that are conserved in the development of all organisms. More specifically, the lab has two main lines of research:
Cytoplasmic flows and cellular asymmetries in the oocyte
Regulation of cell fate and tissue architecture in ovaries and testis
Cytoplasmic flows and cellular asymmetries in the oocyte
Regulation of Cell Fate and Tissue Architecture in Ovaries and Testis
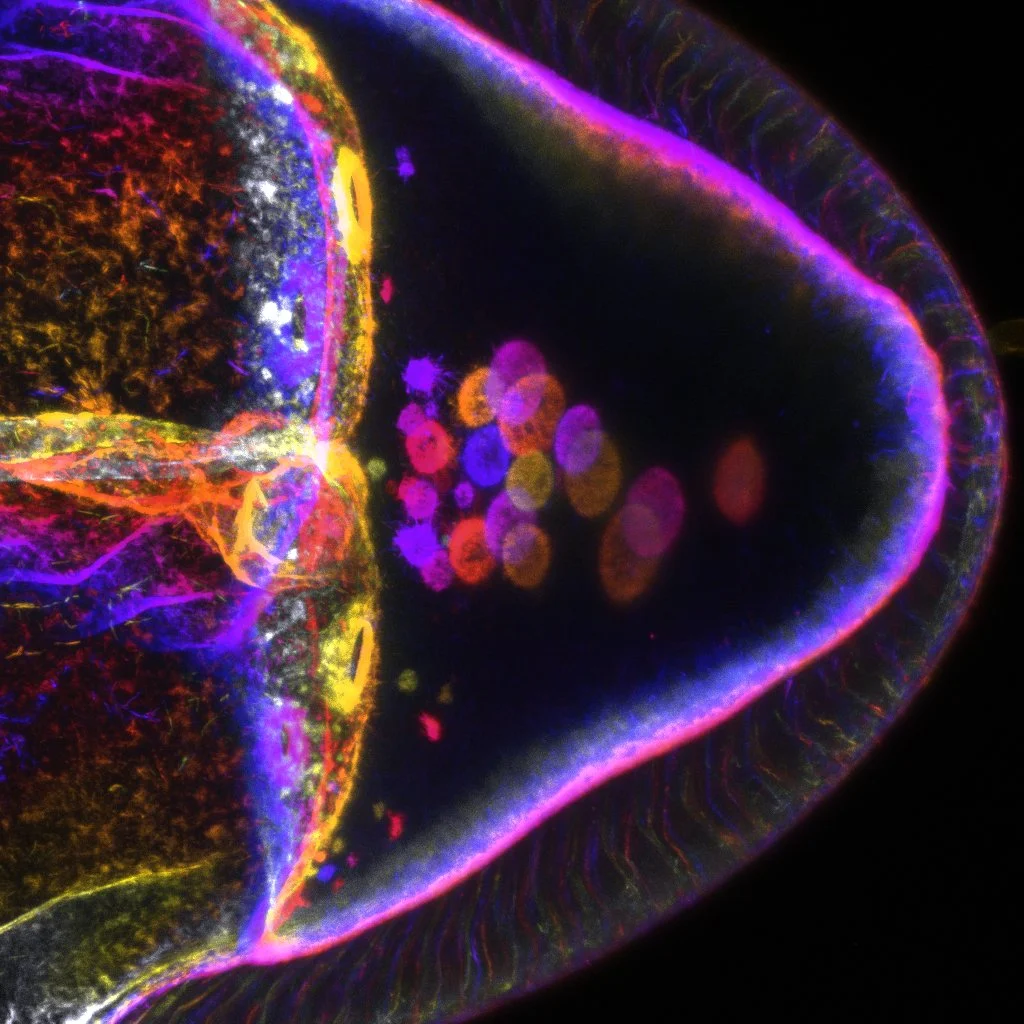
We are investigating cellular asymmetries in the oocyte, and their relation with the fluid mechanical properties of the cytoplasm. In collaboration with physicist Prof. R. Goldstein, we have a unique approach that combines interdisciplinary experimental and theoretical parameters in order to answer these questions. A physicist (Dr. A. Jimenez Dalmaroni) is soon joining the lab. In the oocyte, as the developmental determinants are being asymmetrically localised, motor proteins also induce the vigorous movement of the oocyte cytoplasm, known as cytoplasmic streaming. Cytoplasmic streaming was discovered in 1774, but many fundamental questions have remained unanswered: How does the fluid motion arise? What is the relationship between the oocyte asymmetries and the underlying forces of the observed flows? We have engaged in a comprehensive experimental and theoretical study of fluid dynamical and transport issues, using techniques from microfluidics to functional genetics. This interdisciplinary approach allows us to cover the various aspects of quantitative biological data collection, physical modelling and experimental design required to study the relation between the fluid mechanical properties of the cytoplasm, the cytoskeletons (actin and microtubules) and oocyte polarity. We are also studying how motors achieve specificity in their functions. This work combines traditional genetic and cell biological approaches with state-of-the art analysis of single molecule dynamics.
The second line of research examines the function of the conserved Hippo (Hpo) tumour-suppressor pathway in cell fate and tissue architecture. In brief, the Hpo pathway consists of a cascade of kinases that regulate the transcription of cell growth genes. We (and others) have showed that Hpo also influences cell fate and tissue architecture. However, not much is known about these Hpo functions. Although many upstream regulators of Hpo are characterised, the identity and function of its transcriptional targets are largely unknown. We are addressing these questions combining functional genomic and cell biology approaches.
2.1. Hippo and cell differentiation in the female germline. The polarisation of the oocyte (required for the localisation of the developmental determinants) relies on the Hpo-dependent differentiation of a group of cells, the posterior follicle cells (FCs), which are in direct contact with the oocyte. We aim to understand what makes the posterior FCs become posterior, and how Hpo contributes to this process. To achieve this, we have obtained the genomic profiling of posterior FCs, and we have compared it to the transcriptome in other FCs. We are currently analysing candidate genes, and the preliminary results are very encouraging. In addition, in contrast to wildtype cells, hpo mutant FCs form a multilayered epithelium. We are investigating how the follicular epithelium maintains a monolayer, and how this mechanism is disrupted in hpo mutant cells.
2.2. Hippo and cell differentiation in the male germline. We have recently found that Hpo also controls the fate of the germline stem cells in testis (GSCs). Stem cells are crucial for homeostasis (maintaining populations of highly differentiated cell types), and thus a balance between self-renewal and differentiation must be maintained. Signals from the niche cells (of somatic origin) regulate this balance. However, little is known about how niche cell number and function is regulated. We have observed that GSCs can lose their stem cell-ness and incorporate into the niche as a niche cell. This is rarely observed in young testis, but it is more prominent in older testis. These observations raise the exciting possibility that GSCs contribute to maintaining a “healthy” niche during the known age-related decline in niche function. We have also found that mutating the Hpo pathway in GSCs dramatically increases the events of GSC-to-niche transformation. We aim to further study this novel switch in stem cell fate, and how Hpo regulates it.
Tissue Mechanics in 3D Human Organoids
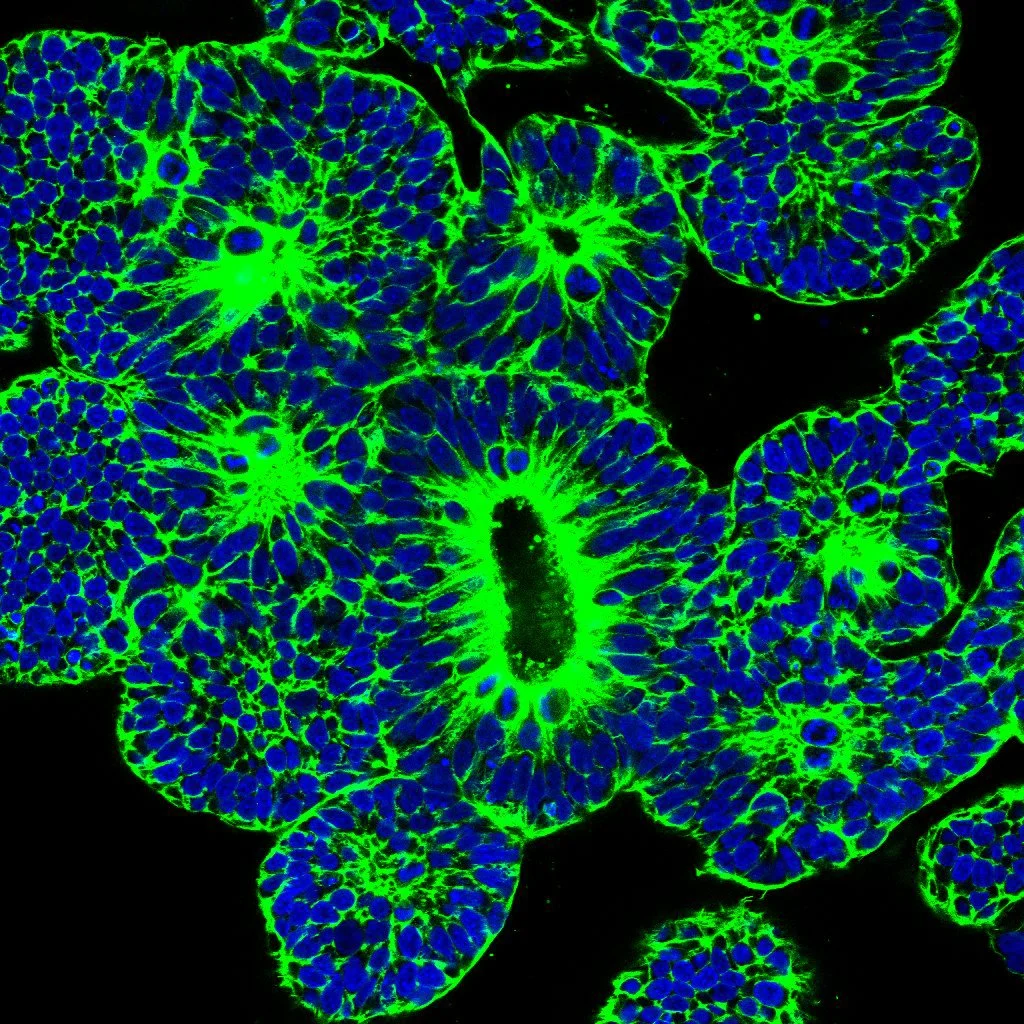
The failure rates of clinical trial for CNS disorders are higher compared to other clinical trials, possibly due to the existence of crucial - not yet understood - differences between pre-clinical models (e.g., mouse) and actual human brains. This raises the question: what makes a human brain unique? One potential factor contributing to our cognitive superiority over other primates is our larger brain size relative to body size, known as the encephalisation quotient, which is directly linked to a higher number of brain cells. To understand these size variations, it is essential to examine early brain development. Most studies investigating the uniqueness of the human brain have primarily focused on neurogenesis, neuronal differentiation, and connectome (mapping the functional and structural connections within the brain organoid). However, limited knowledge exists regarding how morphogenesis and tissue architecture may contribute to the fundamental framework of a larger brain.
Recent advancements in generating organoids from induced pluripotent stem cells (iPSCs) have enabled the study of a variety of developmental processes. It can be argued that since organoids develop following intrinsic developmental programmes, the tissue morphology resembles well the architecture of the organ. Relevant for this proposal is the fact that culturing human cerebral organoids provides a valuable 3D model for investigating human brain development. The formation of the human columnar neuroepithelium (NE) is the first step in the development of the brain. Furthermore, to better understand this process and the impact on brain size there will be used human iPSCs-derived cerebral organoids, with special focus on the regulation of columnar cell shape and forces of the NE, an important event as NE cell shape contributes to human brain size. In an interdisciplinary approach, there will be combined bioengineering, cell biophysics and functional genetics to study the biophysical principles governing NE development.